As of February 6th Fairfield County is currently in the Medium Category.
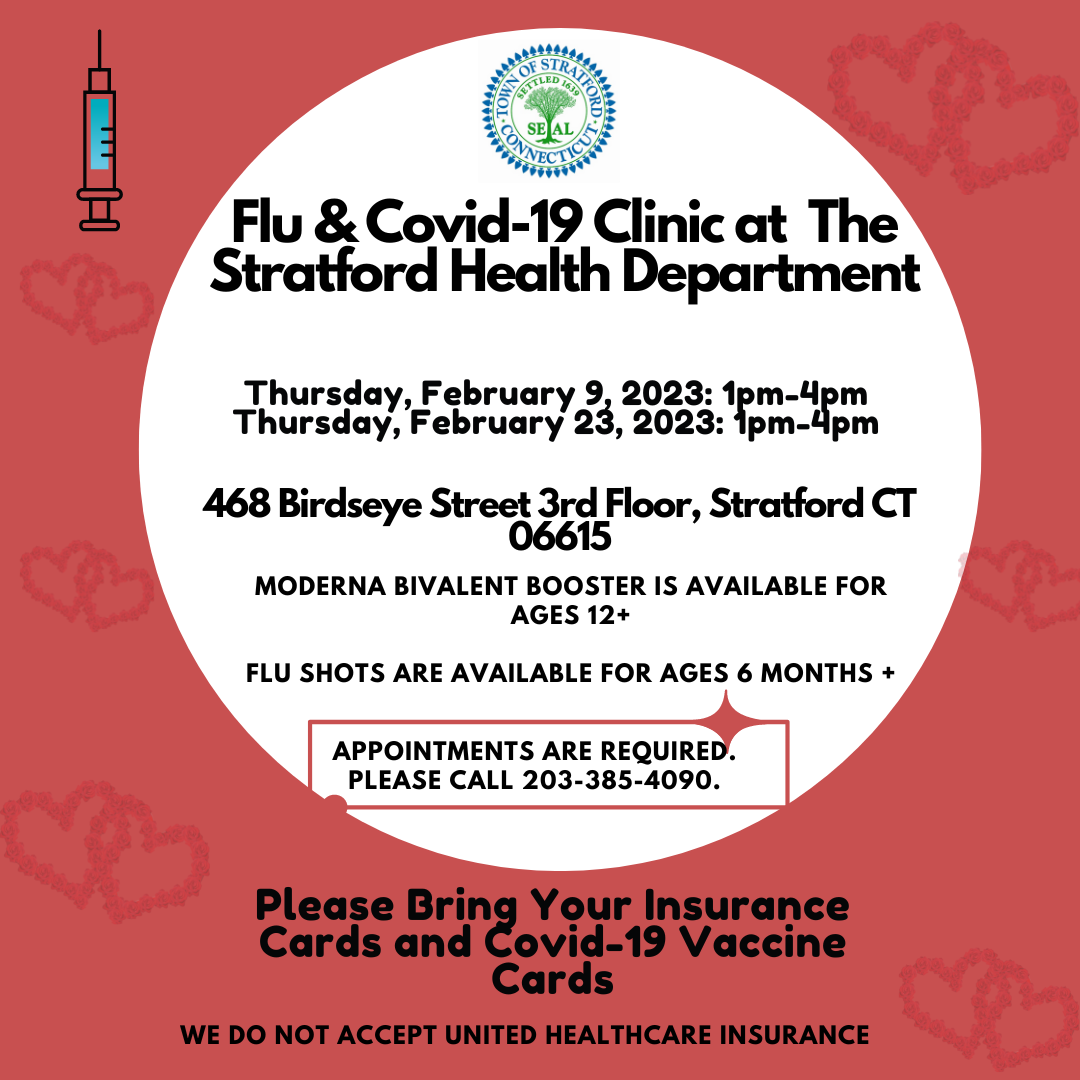
The Stratford Health Department has scheduled a Covid 19/flu clinic for Thursday, February 9th, at Birdseye School, 468 Birdseye Street, 3rd Floor.
A Covid/Flu clinic is also scheduled for Thursday, February 23rd from 1 p.m. to 4 p.m.
Get free Covid tests. Every U.S. household is eligible to order 4 free at-home COVID-19 tests. Order Free At-Home Tests at: https://www.covid.gov/tests
Need help placing an order for your at-home tests?
Call 1-800-232-0233 (TTY 1-888-720-7489).
CONFIRMED CASES OF COVID-19 IN STRATFORD February 6th
Number of Cases for the Past Seven Days: 38
Percent Positivity for the Past Seven Days: 11.4%
Total Cumulative Cases: 16,799
There have been 220 deaths to date. STRATFORD VACCINE UPDATE
The state is releasing information about how many individuals are vaccinated in all Connecticut communities. As of February 1, 2023, 80.32% of Stratford residents have received at least one dose of the COVID-19 vaccine. If you haven’t yet gotten boosted, consider now.
Stratford clinics have dispensed 16,893 vaccines to date. It’s important to keep in mind that we are part of a larger regional and statewide vaccination network and effort.
Stratford does not vaccinate ONLY Stratford residents – many of residents and first responders have been vaccinated at locations outside Stratford, and conversely, many from outside of Stratford have been vaccinated here.
If you have any questions or concerns regarding the COVID-19 vaccine please call us. We are happy to discuss the vaccine and any concerns you may have. You can contact us by email at health@townofstratford.com or by phone at 203-385-4090.
Stratford, and conversely, many from outside of Stratford have been vaccinated here.
BIVALENT BOOSTER FACTS *UPDATE
The The FDA authorized both the Moderna and Pfizer-BioNTech bivalent boosters. The bivalent boosters combine the original vaccine with protection against the newest omicron versions to increase cross-protection against multiple COVID-19 variants. Updated COVID-19 vaccines are formulated to protect against some of the more recently circulating viruses. Most importantly, COVID-19 vaccines are critical to providing ongoing protection as immunity wanes and the virus continues to mutate.
The FDA expanded the age authorization for both Modern and Pfizer bivalent boosters. The CDC is now recommending that everyone aged 6 months and older receive the updated bivalent booster. Children ages 6 months through 5 years who previously completed a Moderna primary series are eligible to receive a Moderna bivalent booster 2 months after their final primary series dose. Children ages 6 months through 4 years who are currently completing a Pfizer primary series will receive a Pfizer bivalent vaccine as their third primary dose.
The FDA expanded the age authorization for both Modern and Pfizer bivalent boosters. The CDC is now recommending that everyone aged 5 and older receive one dose of the updated bivalent booster.
Moderna bivalent:Individuals 6 years of age and older are eligible for a single booster dose of the Moderna bivalent COVID-19 vaccine if it has been at least two months since they have completed primary vaccination or have received the most recent booster dose with any authorized or approved monovalent COVID-19 vaccine.
Pfizer bivalent:Individuals 5 years of age and older are eligible for a single booster dose of the Pfizer-BioNTech bivalent COVID-19 vaccine if it has been at least two months since they have completed primary vaccination or have received the most recent booster dose with any authorized or approved monovalent COVID-19 vaccine.
The FDA expanded the age authorization for both Modern and Pfizer bivalent boosters. The CDC is now recommending that everyone aged 5 and older receive one dose of the updated bivalent booster.
Pfizer bivalent: Individuals 5 years of age and older are eligible for a single booster dose of the Pfizer-BioNTech bivalent COVID-19 vaccine if it has been at least two months since they have completed primary vaccination or have received the most recent booster dose with any authorized or approved monovalent COVID-19 vaccine.
CDC Recommends Moderna for those Aged 6-17
The Centers for Disease Control and Prevention (CDC) endorsed the CDC Advisory Committee on Immunization Practices’ (ACIP) recommendation that Moderna’s COVID-19 vaccine be used as an option for children ages 6 through 17 years, in addition to its already recommended use in children 6 months through 5 years and adults 18 years and older. The ACIP recommendation comes after a thorough review of the scientific evidence demonstrating safety and efficacy and supports the use of the vaccine among those 6 through 17 years of age. CDC recommends that Moderna COVID-19 vaccine be used for individuals 6 through 17 years of age to better protect them from COVID-19.
COVID-19 Vaccine Update
The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) voted to recommend children 6 months through 5 years of age receive a COVID-19 vaccine. The CDC now recommends that all children 6 months through 5 years of age receive either the Pfizer-BioNTech or Moderna COVID-19 vaccine to better protect them from COVID-19.
All children, including those who have already had COVID-19, should get vaccinated. Although most children have only mild symptoms when infected, COVID-19 can cause some children to become very sick, even to the point of requiring hospitalization or even death.
The approval of COVID-19 vaccines for children as young as 6-months old is another major step forward in the overall COVID-19 vaccine roll-out. Parents have many options for where to get a COVID-19 vaccine for their child including:
Pediatricians’ offices: Hundreds of pediatricians will be administering COVID-19 vaccines across Connecticut.